[vc_row css_animation=”” row_type=”row” use_row_as_full_screen_section=”no” type=”full_width” angled_section=”no” text_align=”left” background_image_as_pattern=”without_pattern”][vc_column][vc_empty_space height=”150px”][vc_column_text]
Do you need these due to Unique Device Identification (UDI) Compliance?
 History of the Worry Doll: The indigenous people from the Highlands in Guatemala created Worry Dolls many generations ago as a remedy for worrying. (source: shamansmarket) |
Let us be the remedy to your worries.Complete the form below and let’s chat about UDI Compliance and how to get your medical devices ready. |
AB&R® is here to take away your worries!
Preparing for Unique Device Identification (UDI) Compliance deadlines requires a project plan that has been thought out. An individual or team of individuals may be needed to take ownership of the UDI project and manage it accordingly. In some cases, it is best to seek outside resources for help.

AB&R® HAS TECHNOLOGY SOLUTIONS THAT MAKE IT EASY TO MEET FDA UNIQUE DEVICE IDENTIFICATION (UDI) STANDARDS
Lessons learned from medical device manufacturers and providers just like you working towards UDI Compliance.
1. Have a contingency plan:
The FDA’s Global UDI Database (GUDID), used for label and product data is a technology and like any technology, there can be glitches. Keep this in mind by formulating contingency plans in the event that there is a temporary shutdown of the GUDID.
2. Define the master:
It’s important to maintain a template with the components needed for the GUDID. In most cases, you’re going to have many different systems where data may live and be sourced from with several team members working within them. Communication is key and creating documentation that can assist training efforts and standardize the master plan will reduce errors.
3. See the big picture:
Keep in mind these standards will eventually be global. Think through your action plan and avoid having to start over in the future when your overseas locations move towards UDI compliance by involving them now.
4. Don’t forget that packaging is affected:
From individual boxes to cases, all will need to be UDI compliant. Unless the products inside the case will be staying there until they are used, if you sell products boxed by the case, both levels of packaging will require a UDI.
5. The upside to UDI:
UDI is expensive, but there are benefits to be realized. One example is the ability to forecast and plan based on the demand of their customers. Perhaps the most important factor to keep in mind is the benefit of preventing or avoiding product failures before they become more serious. In addition, it can generate information into what kinds of product attributes work best for a particular patient. This can be used to help market products in a world of personalized medicine and inform research and development efforts as providers become more discerning about new product innovations.
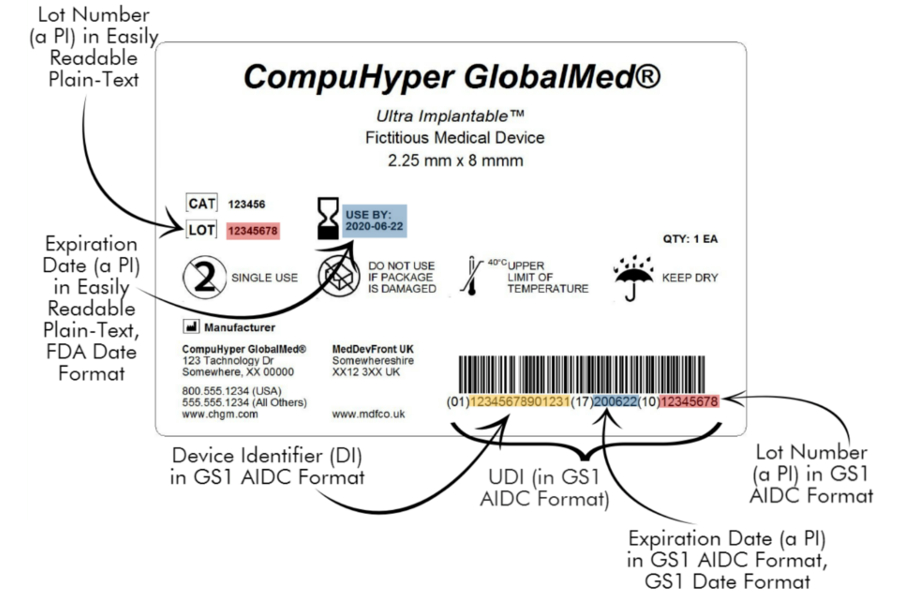
Components of a UDI Label:
2018 deadline is approaching:
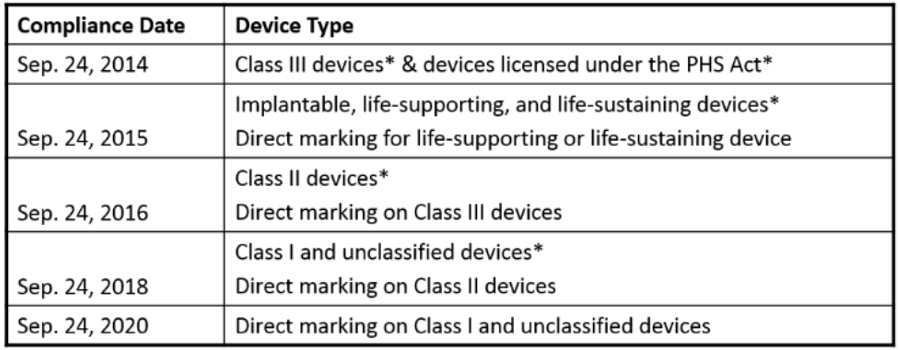
Specifics to September 24, 2018 Deadline:
| A class II device that is required to be labeled with a UDI must bear a UDI as a permanent marking on the device itself if the device is a device intended to be used more than once and intended to be reprocessed before each use. § 801.45. |
| The labels and packages of class I medical devices and devices that have not been classified into class I, class II, or class III must bear a UDI. § 801.20. Dates on the labels of all devices, including devices that have been excepted from UDI labeling requirements, must be formatted as required by § 801.18. |
| Data for class I devices and devices that have not been classified into class I, class II, or class III that are required to be labeled with a UDI must be submitted to the GUDID database. § 830.300. Class I stand-alone software must provide its UDI as required by § 801.50(b). |
Need more?
Read Steps for successful UDI Compliance or visit the FDA website for full guidelines and details.[/vc_column_text][/vc_column][/vc_row]
